The flexibility of the buffer solution to face up to variations in pH when a little bit amount of acid or foundation is included is generally known as buffer motion.
It's used as cleansing agent .Nitric acid is treated with challenging h2o and convert into soft drinking water by precipitating the calcium and magnesium impurity.
Buffers are characterized by two parameters, buffer variety and buffer capability. Buffer assortment would be the interval of pH values in excess of which a buffer can reliably neutralize extra exterior acids/bases. It truly is equivalent to p
Improve the post using your abilities. Add on the GeeksforGeeks Local community and support generate superior Mastering methods for all.
The harmony delivers action-by-action Recommendations so analysts can certainly keep an eye on the place They can be inside the buffer preparation process.
Buffering Capability may be the property of solutions that's represented from the image β. It really is described because the ratio of your moles of acid or base needed to alter the pH in the solution by one and also the pH transform and the volume of buffers.
The three parts of the next example illustrate the adjust in pH that accompanies the addition of foundation to a buffered solution of the weak acid also to an unbuffered solution of a robust acid.
The level of dissociation can differ with temperature. Your buffer solution need to hence be prepared at precisely the same temperature at which you'll perform your analyses. Also Ensure that the temperature at which you calibrate the electrode is the same as the temperature at buffer solution preparation which you measure.
Buffer solutions are acknowledged to get resistant to pH improvements. A buffer solution’s pH, on the other hand, can differ based upon the amount of potent acid or strong base is included.
a dramatic modify in pH. If we attempt including a little degree of foundation for the buffer solution, the weak acid that's current will neutralize the extra base. So during the balanced Internet ionic equation, hydroxide anions react with acetic acid to variety the acetate anion and water.
As an example, if a buffer is formed from the weak acid HA and its conjugate foundation A–, any proton transfer that occurs yields products that are just like the reactants, a course of action generally known as an identity response
By clicking “Take All Cookies”, you agree to the storing of cookies in your machine to reinforce web-site navigation, review web site usage, and assist inside our marketing efforts.
I believe Anything you’re attempting to ask is why does the conjugate base neutralize most of the included acid if it’s only a weak acid. A buffer solution can be website an equilibrium involving a weak acid and its conjugate base primarily.
Bases PH are usually greater than 7.Its significant PH residence form the foam with the h2o so it can be used within the manufacturing of soap for eliminating the Filth from outfits.
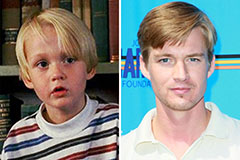 Mason Gamble Then & Now!
Mason Gamble Then & Now! Yasmine Bleeth Then & Now!
Yasmine Bleeth Then & Now!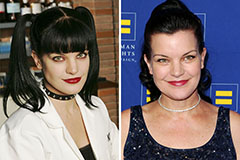 Pauley Perrette Then & Now!
Pauley Perrette Then & Now! Lisa Whelchel Then & Now!
Lisa Whelchel Then & Now! Meadow Walker Then & Now!
Meadow Walker Then & Now!